Lynne Oland, Ph.D.

Undergraduate Neuroscience and Cognitive Science Program
I serve as the faculty Director of this program, which was approved in May of 2011 and now has grown to serve about 650 students. Ours is a robust and rigorous program with multiple opportunities for students to customize their program.33% of majors said that the NSCS program was the reason they came to the University of Arizona or that the existence of the program strongly influenced their decision. 41% of our students are honors students, students graduating in 2018 had an average GPA of 3.51, and about 40% are directly involved in laboratory research. About 25% are Hispanic students, 32% are first-generation students, and about 80% are Arizona residents. 49% were involved in some sort of outreach. We find that a consistent strength of the program is that we have fostered a strong and tight knit community.
Research Interests:
- neuron-glia interactions in the developing nervous system
- role of glial cells in modulating neuronal activity at synapses
Glial cells once were relegated to the status of supporting cells for neurons. Recent research, however, has cast the glia in a new light, revealing them to be involved with neurons in complex interactions that strongly affect the viability, growth, and functioning of the neurons. During development, glial cells have especially important roles in the survival and migration of neurons, guidance of axons, and formation of neuronal branching patterns. But most interestingly, our work and that of other labs has shown that the neurons in turn influence glial cell development, suggesting that a dynamic interaction between the two cell classes is a necessary element in correct development of the nervous system. Because recognition that these neuron-glial cell interactions can have such profound effects is so recent, our knowledge of the repertoire of interactions undoubtedly is incomplete and our understanding of the underlying mechanisms is still nascent. Similarly, though it is widely recognized that glial cells and neurons interact in the more mature brain to modulate neuronal function, the mechanisms remain poorly understood, but clearly involve modulation of synaptic interactions and plasticity.
Our lab was co-directed by my longstanding colleague, Regents Professor Leslie Tolbert.
Key findings from our work
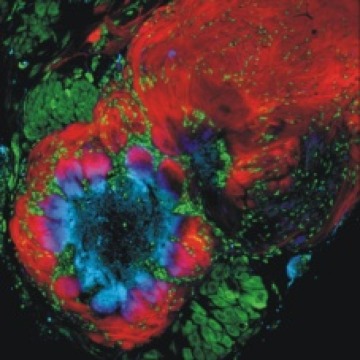
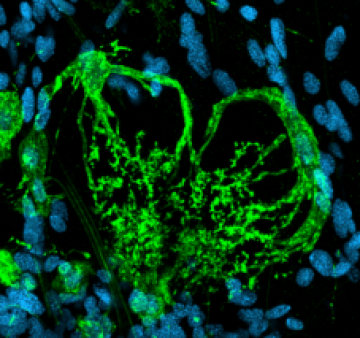
1. Role of glial cells in development of the olfactory (antennal) lobe of the moth Manduca sexta.
During the period of olfactory axon ingrowth, in metamorphosis, the axons induce the formation of olfactory glomeruli, which appear in virtually all primary olfactory centers across the animal kingdom. The axons pierce the glial border that surrounds the nascent olfactory neuropil and the terminal branches of axons carrying the same olfactory receptors cluster into protoglomeruli.These protoglomerular structures are stabilized by the glial cells that migrate into the neuropil between the axonal clusters and extend their processes around them to form a nearly complete envelope around each. In this system, axonal protoglomeruli set the template for the mature glomerular array. In the absence of glial cells, the protoglomeruli form but are not stabilized, and the projection neurons (equivalent to mitral cells) fail to develop their normal tufted arbors. Nitric oxide released by the ingrowing axons triggers glial cell migration to surround the developing glomeruli.
Whole-cell recordings from these glial cells showed that glial migration is dependent on voltage-activated calcium currents that were induced by the ingrowing sensory axons. In addition, whole-cell recordings showed that the receptor axons also influence the development of glial potassium currents; in their absence, the potassium current profile in antennal lobe glial cells remains in its immature configuration.
Figure 1
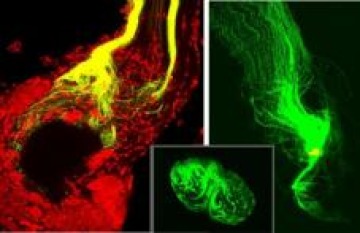
2. Role of glial cells in guiding and sorting ingrowing olfactory sensory axons to bring axons targeting the same glomeruli together
The axon-sorting region is heavily populated by glial cells that arise form the population of central glia and migrate out into the region of the nerve just outside the antennal lobe. Axons entering this region undergo a sorting process that changes their topographic organization into a chemotopic one. When the glial population is severely reduced just before the axon-ingrowth period, axons fail to sort properly and may even bypass their normal antennal-lobe target.
Neuronal activity turned out not to be necessary for the formation of glomerular architecture. Olfactory axon ingrowth, axonal fasciculation and glial migration appear to depend on several cell-surface and extracellular signaling molecules, including fascclin II, tenascin, and both EGF and FGF receptors. When these latter molecules are blocked, axon ingrowth stalls in the sorting zone and bundles are disrupted or fail to form.
Figure 2
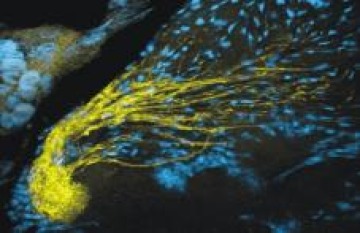
3. Complex glial cells
Each glomerulus is surrounded by about 75 to 100 simple, roughly spindle-shaped glial cells that form the glial envelope, but do not penetrate the neuropil of the glomerulus.In addition, there are 5 or so complex glial cells whose processes reach to the base of the glomerulus where they branch extensively. These cells express a GABA transporter (as do the sorting-zone glial cells) and are likely to play a role in modulating synaptic activity within the glomeruli.
Figure 3
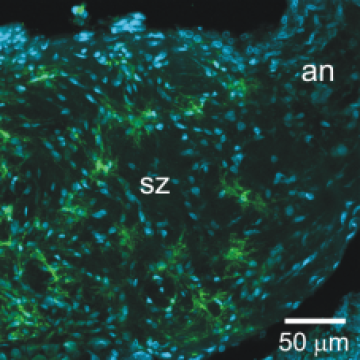
4. Development of the antennal nerve glial network
In the mature antennal (olfactory) nerve, glial cells form a network of processes that ensheathe bundles of 60-100 axons. Most of these glial cells arise in the periphery and migrate into the nerve just after the outgrowing olfactory axons, some continuing to divide during this process. Eventually they extend processes to form the network. These glial cells have both potassium and calcium currents and blocking the glial R-like calcium current blocks migration of the peripheral glial cells. Normal development of the calcium current depends on neuronal activity.
5. Neuron-glia interactions that modulate synaptic function
Several years ago, we turned to Drosophila to explore how and to what effect the astrocyte-like glial cells interact with synapses to alter their function. We chose the 3rd instar larval stage in which both the morphology and physiological behavior of certain motor neuron circuits in the ventral nerve cord had been studied in detail. Although our electron microscopic reconstructions of astrocyte process relationship to synapses revealed that Drosophila synapses were not ensheathed by glial processes and in general were further from synapses than their vertebrate counterparts, no synapse was further than 1 mm from a glial process. Then graduate student Sarah MacNamee collected the first whole-cell recordings from Drosophila astrocytes and found their intrinsic membrane properties to be shared with astrocytes of highly divergent species. Optogenetic activation of a group of glutamatergic pre-motor neurons showed that astrocytes respond with a glutamate transporter current (Eaat1) and blockade of the transporter affected the decay of post-synaptic motor neuron currents. These glial cells also were often dye-coupled.
We also generated a library of fluorescently-labeled astrocytes, which showed that the population has considerable variability in shape and size, that there are many instances of bilateral symmetry in shape, that like their vertebrate counterparts there is little overlap in territory between adjacent astrocytes, and that a given astrocyte’s domain can cover more than one neuronal functional domain.
Selected Publications:
Oland LA, Tolbert LP (1987) Glial patterns during early development of antennal lobes of Manduca sexta: a comparison between normal lobes and lobes deprived of antennal axons. Journal of Comparative Neurology 255:196-207.
Oland LA, Orr G, Tolbert LP (1990) Construction of a protoglomerular template by olfactory axons initiates the formation of olfactory glomeruli in the insect brain. Journal of Neuroscience 10:2096-2112.
Baumann PM, Oland LA, Tolbert LP (1996) Glial cells stabilize axonal protoglomeruli in the developing olfactory lobe of the moth Manduca sexta. Journal of Comparative Neurology 373:118-128.
Rössler WR, Oland LA, Higgins MR, Hildebrand JG, Tolbert LP (1999) Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. Journal of Neuroscience 19:9865-77.
Lohr C, Oland LA, Tolbert LP (2001) Olfactory receptor axons influence the development of glial potassium currents in the antennal lobe of the moth Manduca sexta. Glia 36:309-320.
Lohr C, Tucker S, Oland LA, Tolbert LP (2002) Development of depolarization-induced calcium transients in insect glial cells is dependent on the presence of afferent axons. Journal of Neurobiology 52:85-98.
Gibson NJ, Tolbert LP, Oland LA (2009) Roles of specific membrane lipid domains in EGF receptor activation and cell adhesion molecule stabilization in a developing olfactory system. PLoS One 4:e7222.
Oland LA, Gibson NJ, Tolbert LP (2010) Localization of a GABA transporter to glial cells in the developing and adult olfactory pathway of the moth Manduca sexta. Journal of Comparative Neurology 518:815-38.
Koussa MA, Tolbert LP, Oland LA. (2011) Development of a glial network in the olfactory nerve: role of calcium and neuronal activity. Neuron Glia Biology 2:1-17.
Gibson NJ, Tolbert LP, Oland LA (2012) Activation of glial FGFRs is essential in bidirectional axon-glia signaling during development of the moth olfactory system. PLoS One 7: e33828.
MacNamee SE, Liu KE, Gerhard S, Tran C, Fetter RD, Cardona A, Tolbert LP, Oland LA (2016) Astrocyte glutamate transport regulates a Drosophila pre-motor synapse lacking astrocyte ensheathment. Journal of Comparative Neurology 524:1979-1998. Doi: 10.1002/cne.24016