Haijiang Cai, Ph.D.

Office: Rm 427 Gould-Simpson
Mailing Address:
Department of Neuroscience
University of Arizona
GS 611, 1040 E. 4th St. PO Box 210077
Tucson, AZ 85721-0077
Documents
B.S. USTC, 2001; Ph.D. USC, 2007; Postdoc, Caltech, 2008-2015.
RESEARCH
We are studying neural circuit mechanisms of animal behaviors in health and disease, with a focus on understanding how the neural circuits regulate eating and emotional behaviors such as fear, anxiety, and depression.
We use multidisciplinary approaches including transgenic mice, optogenetics, chemogenetics, in vivo calcium imaging, various behavioral assays, brain slice electrophysiology, virus and non-virus based neuronal tracing, stereotaxic injection and surgery, immunohistology and microscopy imaging.
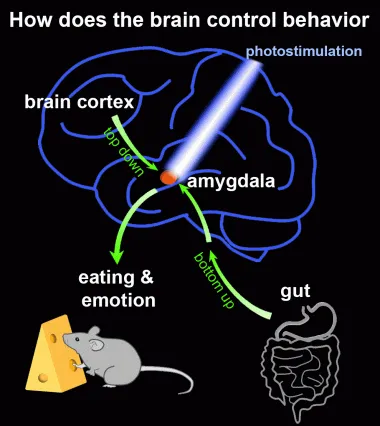
2. Brain circuits for gut-brain communication. Satiation signals, such as cholecystokinin (CCK), are released in the gastrointestinal (GI) tract during a meal. These signals activate the vagus nerve, which transmits information to the brainstem’s nucleus tractus solitarius (NTS), and subsequently to the parabrachial nucleus (PBN). The PBN then relays this information to the central amygdala (CeA) to suppress eating. Interestingly, CeA neurons serve as the first inhibitory relay in this pathway. As a result, functional circuit mapping using c-Fos cannot directly trace activity downstream of the CeA. The genetic identification of CeA neurons has provided us with a powerful tool to determine the downstream brain regions influenced by the CeA and to investigate the neural circuit mechanisms underlying gut-brain communication.
3. Eating and emotion circuits in diseases. Obesity is a serious global health issue in modern society. Do the neural circuits that regulate emotion and acute eating behaviors also contribute to the long-term homeostasis of energy balance, metabolism, and body weight? Eating disorders such as anorexia nervosa are associated with abnormal eating behaviors and emotional disturbances. Our studies have shown that neurons in the CeA and BNST are required for the development of anorexia in an animal model. Understanding how these neurons regulate anorexia is a critical to develop therapies. Many genetic disorders such as Prader-Willi Syndrome are associated with abnormal appetite-related behaviors. We are also studying how neural circuits in the CeA contribute to impaired eating behaviors in these conditions. [Arizona Public Media (AZPM): Episode 194: Researching eating disorders with lab mice]
Selected publications (click here for the complete list)
- Neural circuits regulation of satiation. Appetite. 2024 May 25;200:107512. doi: 10.1016/j.appet.2024.107512. [Epub ahead of print] Review. PubMed PMID: 38801994.
- Development of activity-based anorexia requires PKC-δ neurons in two central extended amygdala nuclei. Cell Rep. 2024 Mar 26;43(3):113933. doi: 10.1016/j.celrep.2024.113933. Epub 2024 Mar 8. PubMed PMID: 38460131.
News & Media: UA News | EDReferral | AZPM: Tracing the cause of eating disorders - Dissecting a disynaptic central amygdala-parasubthalamic nucleus neural circuit that mediates cholecystokinin-induced eating suppression. Mol Metab. 2022 Jan 20;58:101443. doi: 10.1016/j.molmet.2022.101443. [Epub ahead of print] PubMed PMID: 35066159.
News & Media: UA News | Daily Wildcat / AZPM | Arizona PBS | KVOA News 4 Tucson | ScienceDaily | EurekAlert! | Neuroscience News | Brainerd Dispatch | Times Now | - Zhang-Molina C, Schmit MB, Cai H. Neural Circuit Mechanism Underlying the Feeding Controlled by Insula-Central Amygdala Pathway. iScience. 2020 Apr 5;23(4):101033. doi: 10.1016/j.isci.2020.101033. [Epub ahead of print] PubMed PMID: 32311583; PubMed Central PMCID: PMC7168768.
- Wang Y, Kim J, Schmit MB, Cho TS, Fang C, Cai H. A bed nucleus of stria terminalis microcircuit regulating inflammation-associated modulation of feeding. Nat Commun. 2019 Jun 24;10(1):2769. doi: 10.1038/s41467-019-10715-x. PubMed PMID: 31235690.
News & Media: UA News | Daily Wildcat | Brain & Behavior Research Foundation News | KGUN-TV 9 News | AZPM | ScienceDaily | EurekAlert! | Neuroscience News | Daily Mail | Genetic Engineering & Biotechnology News - Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014 Sep;17(9):1240-8. doi: 10.1038/nn.3767. Epub 2014 Jul 27. PubMed PMID: 25064852; PubMed Central PMCID: PMC4146747.
News & Media: New York Times: A mouse switch turns off appetite | BBC: Brain cells can suppress appetite, study in mice shows - Haubensak W, Kunwar PS*, Cai H*, Ciocchi S*, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010 Nov 11;468(7321):270-6. doi: 10.1038/nature09553. PubMed PMID: 21068836; PubMed Central PMCID: PMC3597095. *equal contribution
- Cai H, Reim K, Varoqueaux F, Tapechum S, Hill K, Sørensen JB, Brose N, Chow RH. Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19538-43. doi: 10.1073/pnas.0810232105. Epub 2008 Nov 25. PubMed PMID: 19033464; PubMed Central PMCID: PMC2614796.
TEACHING
- NROS 318 (Spring 2018- ): Fundamental Principles in Systems Neuroscience
- NROS 418 Online (Fall 2022-2024, Spring 2025): Fundamental Principles in Systems Neuroscience
- NROS 435 (Fall 2025- ): Complex Behavioral, Cognitive and Emotional Disorders
- NRSC 560 (Spring 2017- ): Systems Neuroscience. Neuroscience GIDP. Course coordinator: Andrew Fuglevand.
- NRSC 588 (Fall 2024- ): Principles of Cellular and Molecular Neurobiology. Course coordinator: Martha C Bhattacharya.
LAB MEMBERS
- Masa Miscevic, Graduate Student, Physiological Sciences GIDP
- Marco Conteras, Research Scientist
- Anna Fang, Research Specialist
- Emma Stewart-Nava, Undergraduate Student
- Brady Rouintree, Undergraduate Student
- Ohm Patel, Undergraduate Student
- Samuel Rautenberg, Undergraduate Student
Lab Alumni
- Postdocs:
Yong Wang (2016-2018), Marina Rodriguez Sanchez (2021), Shivani Mann (2021-2024) - Graduate Students:
Wesley Schnapp (2020-2024, Neuroscience GIDP), Matthew Schmit (2017-2024, Neuroscience GIDP, Postdoc 2025)
Andrew Tubbs (2016 rotation), Hannah Dollish (2017 rotation), Gowri Somasekhar (2020 rotation), Emily McDonald (2022 rotation), Israel Ispuro (2022 rotation), Jean Dill (2024 rotation), Justin Carter (2024 rotation), Briceyda Limon Ovalles (2025 rotation) - Technicians:
JungMin Kim (2016-2018), Ross Mansouri-Rad (2017-2018), Mauricio Serna (2021) - Undergraduate Students:
John Kim (2016), Alison Kath (2016), Tiffany Cho (2017-2019), Tahia Hanseen (2018-2020), Kevin Vo (2018-2020), Mayra Rivera (2019-2021), Gizem Ozturk (2020-2021), Freya Abraham (2020), Veena Raghuraman (2021), Ananya Nigam (2021-2023), Hannah Vu (2021-2023), Cassidy Johnson (2022-2024), Nada Elnady (2023), Sayujya Timilsena (2023-2024), Elise Bouchal (2020-2024), Vivianna Pederson (2023-2024), Brandon Rodriguez (2025, UROC), Alice Miranda (2024-2025) - High School Students:
Makenna Anderson (2017-2018, UHS), Braydon Kim (2024, Keys program), Korey C Schneider (2025, Keys program), Avani Gandivalasa (2025, Keys program)

Cai lab, 2018
GRADUATE PROGRAM AFFILIATION